Dopamine transporter
The dopamine transporter (DAT, also sodium-dependent dopamine transporter) is a membrane-spanning protein coded for in humans by the SLC6A3 gene (also known as DAT1), that pumps the neurotransmitter dopamine out of the synaptic cleft back into cytosol. In the cytosol, other transporters sequester the dopamine into vesicles for storage and later release. Dopamine reuptake via DAT provides the primary mechanism through which dopamine is cleared from synapses, although there may be an exception in the prefrontal cortex, where evidence points to a possibly larger role of the norepinephrine transporter.[5]
DAT is implicated in a number of dopamine-related disorders, including attention deficit hyperactivity disorder, bipolar disorder, clinical depression, eating disorders, and substance use disorders. The gene that encodes the DAT protein is located on chromosome 5, consists of 15 coding exons, and is roughly 64 kbp long. Evidence for the associations between DAT and dopamine related disorders has come from a type of genetic polymorphism, known as a variable number tandem repeat, in the SLC6A3 gene, which influences the amount of protein expressed.[6]
Function
[edit]DAT is an integral membrane protein that removes dopamine from the synaptic cleft and deposits it into surrounding cells, thus terminating the signal of the neurotransmitter. Dopamine underlies several aspects of cognition, including reward, and DAT facilitates regulation of that signal.[7]
Mechanism
[edit]DAT is a symporter that moves dopamine across the cell membrane by coupling the movement to the energetically-favorable movement of sodium ions moving from high to low concentration into the cell. DAT function requires the sequential binding and co-transport of two Na+ ions and one Cl− ion with the dopamine substrate. The driving force for DAT-mediated dopamine reuptake is the ion concentration gradient generated by the plasma membrane Na+/K+ ATPase.[8]
In the most widely accepted model for monoamine transporter function, sodium ions must bind to the extracellular domain of the transporter before dopamine can bind. Once dopamine binds, the protein undergoes a conformational change, which allows both sodium and dopamine to unbind on the intracellular side of the membrane.[9]
Studies using electrophysiology and radioactive-labeled dopamine have confirmed that the dopamine transporter is similar to other monoamine transporters in that one molecule of neurotransmitter can be transported across the membrane with one or two sodium ions. Chloride ions are also needed to prevent a buildup of positive charge. These studies have also shown that transport rate and direction is totally dependent on the sodium gradient.[10]
Because of the tight coupling of the membrane potential and the sodium gradient, activity-induced changes in membrane polarity can dramatically influence transport rates. In addition, the transporter may contribute to dopamine release when the neuron depolarizes.[10]
DAT–Cav coupling
[edit]Preliminary evidence suggests that the dopamine transporter couples to L-type voltage-gated calcium channels (particularly Cav1.2 and Cav1.3), which are expressed in virtually all dopamine neurons.[11] As a result of DAT–Cav coupling, DAT substrates that produce depolarizing currents through the transporter are able to open calcium channels that are coupled to the transporter, resulting in a calcium influx in dopamine neurons.[11] This calcium influx is believed to induce CAMKII-mediated phosphorylation of the dopamine transporter as a downstream effect;[11] since DAT phosphorylation by CAMKII results in dopamine efflux in vivo, activation of transporter-coupled calcium channels is a potential mechanism by which certain drugs (e.g., amphetamine) trigger neurotransmitter release.[11]
Protein structure
[edit]The initial determination of the membrane topology of DAT was based upon hydrophobic sequence analysis and sequence similarities with the GABA transporter. These methods predicted twelve transmembrane domains (TMD) with a large extracellular loop between the third and fourth TMDs.[12] Further characterization of this protein used proteases, which digest proteins into smaller fragments, and glycosylation, which occurs only on extracellular loops, and largely verified the initial predictions of membrane topology.[13] The exact structure of the Drosophila melanogaster dopamine transporter (dDAT) was elucidated in 2013 by X-ray crystallography.[14]
Location and distribution
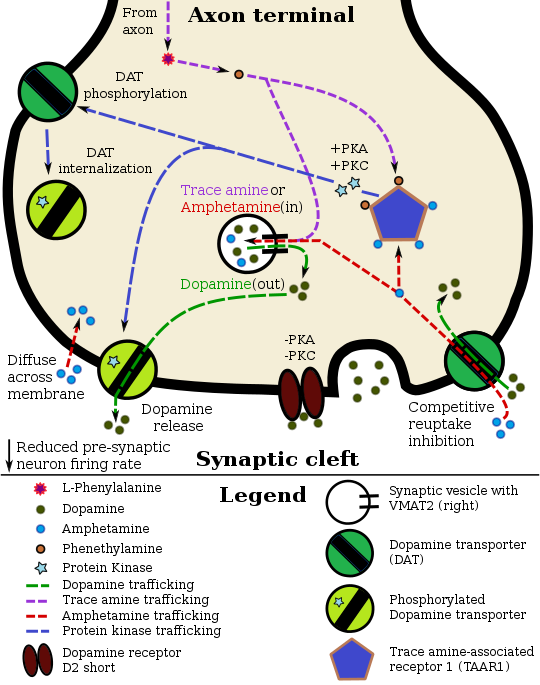
[edit]Pharmacodynamics of amphetamine in a dopamine neuron
|
Regional distribution of DAT has been found in areas of the brain with established dopaminergic circuitry, including the nigrostriatal, mesolimbic, and mesocortical pathways.[22] The nuclei that make up these pathways have distinct patterns of expression. Gene expression patterns in the adult mouse show high expression in the substantia nigra pars compacta.[23]
DAT in the mesocortical pathway, labeled with radioactive antibodies, was found to be enriched in dendrites and cell bodies of neurons in the substantia nigra pars compacta and ventral tegmental area. This pattern makes sense for a protein that regulates dopamine levels in the synapse.
Staining in the striatum and nucleus accumbens of the mesolimbic pathway was dense and heterogeneous. In the striatum, DAT is localized in the plasma membrane of axon terminals. Double immunocytochemistry demonstrated DAT colocalization with two other markers of nigrostriatal terminals, tyrosine hydroxylase and D2 dopamine receptors. The latter was thus demonstrated to be an autoreceptor on cells that release dopamine. TAAR1 is a presynaptic intracellular receptor that is also colocalized with DAT and which has the opposite effect of the D2 autoreceptor when activated;[15][24] i.e., it internalizes dopamine transporters and induces efflux through reversed transporter function via PKA and PKC signaling.
Surprisingly, DAT was not identified within any synaptic active zones. These results suggest that striatal dopamine reuptake may occur outside of synaptic specializations once dopamine diffuses from the synaptic cleft.
In the substantia nigra, DAT is localized to axonal and dendritic (i.e., pre- and post-synaptic) plasma membranes.[25]
Within the perikarya of pars compacta neurons, DAT was localized primarily to rough and smooth endoplasmic reticulum, Golgi complex, and multivesicular bodies, identifying probable sites of synthesis, modification, transport, and degradation.[26]
Genetics and regulation
[edit]The gene for DAT, known as DAT1, is located on chromosome 5p15.[6] The protein encoding region of the gene is over 64 kb long and comprises 15 coding segments or exons.[27] This gene has a variable number tandem repeat (VNTR) at the 3’ end (rs28363170) and another in the intron 8 region.[28] Differences in the VNTR have been shown to affect the basal level of expression of the transporter; consequently, researchers have looked for associations with dopamine-related disorders.[29]
Nurr1, a nuclear receptor that regulates many dopamine-related genes, can bind the promoter region of this gene and induce expression.[30] This promoter may also be the target of the transcription factor Sp-1.
While transcription factors control which cells express DAT, functional regulation of this protein is largely accomplished by kinases. MAPK,[31] CAMKII,[20][21] PKA,[15] and PKC[21][32] can modulate the rate at which the transporter moves dopamine or cause the internalization of DAT. Co-localized TAAR1 is an important regulator of the dopamine transporter that, when activated, phosphorylates DAT through protein kinase A (PKA) and protein kinase C (PKC) signaling.[15][33] Phosphorylation by either protein kinase can result in DAT internalization (non-competitive reuptake inhibition), but PKC-mediated phosphorylation alone induces reverse transporter function (dopamine efflux).[15][34] Dopamine autoreceptors also regulate DAT by directly opposing the effect of TAAR1 activation.[15]
The human dopamine transporter (hDAT) contains a high affinity extracellular zinc binding site which, upon zinc binding, inhibits dopamine reuptake and amplifies amphetamine-induced dopamine efflux in vitro.[35][36][37] In contrast, the human serotonin transporter (hSERT) and human norepinephrine transporter (hNET) do not contain zinc binding sites.[37] Zinc supplementation may reduce the minimum effective dose of amphetamine when it is used for the treatment of attention deficit hyperactivity disorder.[38]
Biological role and disorders
[edit]The rate at which DAT removes dopamine from the synapse can have a profound effect on the amount of dopamine in the cell. This is best evidenced by the severe cognitive deficits, motor abnormalities, and hyperactivity of mice with no dopamine transporters.[39] These characteristics have striking similarities to the symptoms of ADHD.
Differences in the functional VNTR have been identified as risk factors for bipolar disorder[40] and ADHD.[41][42] Data has emerged that suggests there is also an association with stronger withdrawal symptoms from alcoholism, although this is a point of controversy.[43][44] An allele of the DAT gene with normal protein levels is associated with non-smoking behavior and ease of quitting.[45] Additionally, male adolescents particularly those in high-risk families (ones marked by a disengaged mother and absence of maternal affection) who carry the 10-allele VNTR repeat show a statistically significant affinity for antisocial peers.[46][47]
Increased activity of DAT is associated with several different disorders, including clinical depression.[48]
Mutations in DAT have been shown to cause dopamine transporter deficiency syndrome, an autosomal recessive movement disorder characterized by progressively worsening dystonia and parkinsonism.[49]
Pharmacology
[edit]The dopamine transporter is the target of substrates, dopamine releasers, transport inhibitors and allosteric modulators.[50][51]
Cocaine blocks DAT by binding directly to the transporter and reducing the rate of transport.[12] In contrast, amphetamine enters the presynaptic neuron directly through the neuronal membrane or through DAT, competing for reuptake with dopamine. Once inside, it binds to TAAR1 or enters synaptic vesicles through VMAT2. When amphetamine binds to TAAR1, it reduces the firing rate of the postsynaptic neuron and triggers protein kinase A and protein kinase C signaling, resulting in DAT phosphorylation. Phosphorylated DAT then either operates in reverse or withdraws into the presynaptic neuron and ceases transport. When amphetamine enters the synaptic vesicles through VMAT2, dopamine is released into the cytosol.[15][16] Amphetamine also produces dopamine efflux through a second TAAR1-independent mechanism involving CAMKIIα-mediated phosphorylation of the transporter, which putatively arises from the activation of DAT-coupled L-type calcium channels by amphetamine.[11]
The dopaminergic mechanisms of each drug are believed to underlie the pleasurable feelings elicited by these substances.[7]
Interactions
[edit]Dopamine transporter has been shown to interact with:
Apart from these innate protein-protein interactions, recent studies demonstrated that viral proteins such as HIV-1 Tat protein interacts with the DAT[57][58] and this binding may alter the dopamine homeostasis in HIV positive individuals which is a contributing factor for the HIV-associated neurocognitive disorders.[59]
Ligands and modulators
[edit]Substrates
[edit]- Dopamine[60][61]
- Norepinephrine[60]
- Substrate-type dopamine releasing agents (e.g., amphetamine)[62][61]
- Catecholaminergic activity enhancers (e.g., selegiline, PPAP, BPAP)[63]
- Certain dopaminergic neurotoxins (e.g., MPTP, 6-OHDA)[64][65][66]
Dopamine reuptake inhibitors (DRIs)
[edit]Typical or classical cocaine-like blockers
[edit]- Amfonelic acid[60][67][68]
- Amineptine[69][70][71][72]
- BTCP[73]
- Cocaethylene[74][75]
- Cocaine[76][77]
- JJC8-088[78][79]
- Methylenedioxypyrovalerone (MDPV)[80][81]
- Methylphenidate[76]
- Orphenadrine[82][83]
- Pethidine (meperidine)[84][85][86]
- Pipradrol[87]
- RTI-55[88]
- Troparil (WIN-35065)[76]
- WIN-35428 (β-CFT)[76][88]
These agents may actually act as dopamine releasing agent-esque DAT negative allosteric modulators or "inverse agonists".[76]
Atypical non-psychostimulant blockers
[edit]- Armodafinil[89]
- Benztropine[62][90][91]
- Bupropion[76] (but some potential for cocaine-like actions)[92][93][94][95]
- GBR-12935[76]
- JHW-007[62][88]
- JJC8-091[96][77][97][78]
- Mazindol[76][91][98]
- (S)-MK-26[99][97][100]
- Modafinil[62][89] (but a few cases of misuse)[101]
- Nomifensine[76][91][98] (but some cases of misuse)[102]
- Phenylpiracetam[103][104][105]
- (R)-Phenylpiracetam (MRZ-9547)[99][106][104][107]
- RDS03-94[78]
- Rimcazole[62]
- Sibutramine[76]
- Solriamfetol[108][109]
- Tamoxifen[82][110]
- Tesofensine[76][111]
- Vanoxerine (GBR-12909)[76][62][98]
These agents may actually act as simple competitive DAT blockers without releaser-like "inverse agonist" activity.[76]
Unsorted blockers
[edit]Dopamine releasing agents (DRAs)
[edit]- 2-Aminoindane (2-AI)[117][118]
- 5-Chloro-αMT[119][120]
- α-Ethyltryptamine (αET)[121][122][123]
- α-Methyltryptamine (αMT)[123]
- Aminorex[112][113]
- Amphetamine (both dextro- and levoamphetamine)[76][112]
- Benzylpiperazine (BZP)[113]
- Cathine[124][125]
- Cathinone[62][113]
- Ephedrine[113]
- Lisdexamfetamine (LDX)[126][127]
- Methylenedioxyamphetamine (MDA)[61][113]
- Methylenedioxyethylamphetamine (MDEA)[62]
- Methylenedioxymethamphetamine (MDMA)[112][61]
- Mephedrone[128][129][130]
- Methamphetamine[76][112]
- Methylone[62][130]
- Naphthylisopropylamine (PAL-287)[131][62]
- Octopamine[60][132]
- Pemoline[133][134][135]
- Phenethylamine[62][132]
- Phenmetrazine[112]
- Phentermine[76][112]
- Phenylpropanolamine (PPA)[124][125]
- Pseudoephedrine[124][125]
- Tryptamine[136][119]
- Tyramine[112][113][132]
These agents are also known as substrate-type dopamine releasing agents and as DAT reversers.[76][62]
Allosteric modulators
[edit]Positive allosteric modulators
[edit]Negative allosteric modulators
[edit]See also
[edit]References
[edit]- ^ a b c ENSG00000276996 GRCh38: Ensembl release 89: ENSG00000142319, ENSG00000276996 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000021609 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Carboni E, Tanda GL, Frau R, Di Chiara G (September 1990). "Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals". Journal of Neurochemistry. 55 (3): 1067–70. doi:10.1111/j.1471-4159.1990.tb04599.x. PMID 2117046. S2CID 23682303.
- ^ a b Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. (December 1992). "Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR". Genomics. 14 (4): 1104–6. doi:10.1016/S0888-7543(05)80138-7. PMID 1478653.
- ^ a b Schultz W (July 1998). "Predictive reward signal of dopamine neurons". Journal of Neurophysiology. 80 (1): 1–27. doi:10.1152/jn.1998.80.1.1. PMID 9658025. S2CID 52857162.
- ^ Torres GE, Gainetdinov RR, Caron MG (January 2003). "Plasma membrane monoamine transporters: structure, regulation and function". Nature Reviews. Neuroscience. 4 (1): 13–25. doi:10.1038/nrn1008. PMID 12511858. S2CID 21545649.
- ^ Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG (February 1997). "Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants". The Journal of Neuroscience. 17 (3): 960–74. doi:10.1523/JNEUROSCI.17-03-00960.1997. PMC 6573182. PMID 8994051.
- ^ a b Wheeler DD, Edwards AM, Chapman BM, Ondo JG (August 1993). "A model of the sodium dependence of dopamine uptake in rat striatal synaptosomes". Neurochemical Research. 18 (8): 927–36. doi:10.1007/BF00998279. PMID 8371835. S2CID 42196576.
- ^ a b c d e Cameron KN, Solis E, Ruchala I, De Felice LJ, Eltit JM (November 2015). "Amphetamine activates calcium channels through dopamine transporter-mediated depolarization". Cell Calcium. 58 (5): 457–66. doi:10.1016/j.ceca.2015.06.013. PMC 4631700. PMID 26162812.
One example of interest is CaMKII, which has been well characterized as an effector of Ca2+ currents downstream of L-type Ca2+ channels [21,22]. Interestingly, DAT is a CaMKII substrate and phosphorylated DAT favors the reverse transport of dopamine [48,49], constituting a possible mechanism by which electrical activity and L-type Ca2+ channels may modulate DAT states and dopamine release. ... In summary, our results suggest that pharmacologically, S(+)AMPH is more potent than DA at activating hDAT-mediated depolarizing currents, leading to L-type Ca2+ channel activation, and the S(+)AMPH-induced current is more tightly coupled than DA to open L-type Ca2+ channels.
- ^ a b Kilty JE, Lorang D, Amara SG (October 1991). "Cloning and expression of a cocaine-sensitive rat dopamine transporter". Science. 254 (5031): 578–9. Bibcode:1991Sci...254..578K. doi:10.1126/science.1948035. PMID 1948035.
- ^ Vaughan RA, Kuhar MJ (August 1996). "Dopamine transporter ligand binding domains. Structural and functional properties revealed by limited proteolysis". The Journal of Biological Chemistry. 271 (35): 21672–80. doi:10.1074/jbc.271.35.21672. PMID 8702957.
- ^ Penmatsa A, Wang KH, Gouaux E (November 2013). "X-ray structure of dopamine transporter elucidates antidepressant mechanism". Nature. 503 (7474): 85–90. Bibcode:2013Natur.503...85P. doi:10.1038/nature12533. PMC 3904663. PMID 24037379.
- ^ a b c d e f g h i j k Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry. 116 (2): 164–76. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
- ^ a b c Eiden LE, Weihe E (January 2011). "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Annals of the New York Academy of Sciences. 1216 (1): 86–98. Bibcode:2011NYASA1216...86E. doi:10.1111/j.1749-6632.2010.05906.x. PMC 4183197. PMID 21272013.
- ^ Sulzer D, Cragg SJ, Rice ME (August 2016). "Striatal dopamine neurotransmission: regulation of release and uptake". Basal Ganglia. 6 (3): 123–148. doi:10.1016/j.baga.2016.02.001. PMC 4850498. PMID 27141430.
Despite the challenges in determining synaptic vesicle pH, the proton gradient across the vesicle membrane is of fundamental importance for its function. Exposure of isolated catecholamine vesicles to protonophores collapses the pH gradient and rapidly redistributes transmitter from inside to outside the vesicle. ... Amphetamine and its derivatives like methamphetamine are weak base compounds that are the only widely used class of drugs known to elicit transmitter release by a non-exocytic mechanism. As substrates for both DAT and VMAT, amphetamines can be taken up to the cytosol and then sequestered in vesicles, where they act to collapse the vesicular pH gradient.
- ^ Ledonne A, Berretta N, Davoli A, Rizzo GR, Bernardi G, Mercuri NB (July 2011). "Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons". Front. Syst. Neurosci. 5: 56. doi:10.3389/fnsys.2011.00056. PMC 3131148. PMID 21772817.
Three important new aspects of TAs action have recently emerged: (a) inhibition of firing due to increased release of dopamine; (b) reduction of D2 and GABAB receptor-mediated inhibitory responses (excitatory effects due to disinhibition); and (c) a direct TA1 receptor-mediated activation of GIRK channels which produce cell membrane hyperpolarization.
- ^ "TAAR1". GenAtlas. University of Paris. 28 January 2012. Retrieved 29 May 2014.
• tonically activates inwardly rectifying K(+) channels, which reduces the basal firing frequency of dopamine (DA) neurons of the ventral tegmental area (VTA)
- ^ a b Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG (July 2014). "Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons". Neuron. 83 (2): 404–416. doi:10.1016/j.neuron.2014.05.043. PMC 4159050. PMID 25033183.
AMPH also increases intracellular calcium (Gnegy et al., 2004) that is associated with calmodulin/CamKII activation (Wei et al., 2007) and modulation and trafficking of the DAT (Fog et al., 2006; Sakrikar et al., 2012).
- ^ a b c Vaughan RA, Foster JD (September 2013). "Mechanisms of dopamine transporter regulation in normal and disease states". Trends in Pharmacological Sciences. 34 (9): 489–96. doi:10.1016/j.tips.2013.07.005. PMC 3831354. PMID 23968642.
AMPH and METH also stimulate DA efflux, which is thought to be a crucial element in their addictive properties [80], although the mechanisms do not appear to be identical for each drug [81]. These processes are PKCβ– and CaMK–dependent [72, 82], and PKCβ knock-out mice display decreased AMPH-induced efflux that correlates with reduced AMPH-induced locomotion [72].
- ^ Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, et al. (June 1999). "Immunocytochemical localization of the dopamine transporter in human brain". The Journal of Comparative Neurology. 409 (1): 38–56. doi:10.1002/(SICI)1096-9861(19990621)409:1<38::AID-CNE4>3.0.CO;2-1. PMID 10363710. S2CID 46295607.
- ^ Liu Z, Yan SF, Walker JR, Zwingman TA, Jiang T, Li J, et al. (April 2007). "Study of gene function based on spatial co-expression in a high-resolution mouse brain atlas". BMC Systems Biology. 1: 19. doi:10.1186/1752-0509-1-19. PMC 1863433. PMID 17437647.
- ^ Maguire JJ, Davenport AP (19 July 2016). "Trace amine receptor: TA1 receptor". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. Retrieved 22 September 2016.
- ^ Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM (January 1996). "The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons". The Journal of Neuroscience. 16 (2): 436–47. doi:10.1523/JNEUROSCI.16-02-00436.1996. PMC 6578661. PMID 8551328.
- ^ Hersch SM, Yi H, Heilman CJ, Edwards RH, Levey AI (November 1997). "Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra". The Journal of Comparative Neurology. 388 (2): 211–27. doi:10.1002/(SICI)1096-9861(19971117)388:2<211::AID-CNE3>3.0.CO;2-4. PMID 9368838. S2CID 21202901.
- ^ Kawarai T, Kawakami H, Yamamura Y, Nakamura S (August 1997). "Structure and organization of the gene encoding human dopamine transporter". Gene. 195 (1): 11–8. doi:10.1016/S0378-1119(97)00131-5. PMID 9300814.
- ^ Sano A, Kondoh K, Kakimoto Y, Kondo I (May 1993). "A 40-nucleotide repeat polymorphism in the human dopamine transporter gene". Human Genetics. 91 (4): 405–6. doi:10.1007/BF00217369. PMID 8500798. S2CID 39416578.
- ^ Miller GM, Madras BK (2002). "Polymorphisms in the 3'-untranslated region of human and monkey dopamine transporter genes affect reporter gene expression". Molecular Psychiatry. 7 (1): 44–55. doi:10.1038/sj/mp/4000921. PMID 11803445.
- ^ Sacchetti P, Mitchell TR, Granneman JG, Bannon MJ (March 2001). "Nurr1 enhances transcription of the human dopamine transporter gene through a novel mechanism". Journal of Neurochemistry. 76 (5): 1565–72. doi:10.1046/j.1471-4159.2001.00181.x. PMID 11238740. S2CID 19410051.
- ^ Morón JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, et al. (September 2003). "Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity". The Journal of Neuroscience. 23 (24): 8480–8. doi:10.1523/JNEUROSCI.23-24-08480.2003. PMC 6740378. PMID 13679416.
- ^ Pristupa ZB, McConkey F, Liu F, Man HY, Lee FJ, Wang YT, et al. (September 1998). "Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter". Synapse. 30 (1): 79–87. doi:10.1002/(SICI)1098-2396(199809)30:1<79::AID-SYN10>3.0.CO;2-K. PMID 9704884. S2CID 20618165.
- ^ Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC (March 2005). "Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors". Genomics. 85 (3): 372–85. doi:10.1016/j.ygeno.2004.11.010. PMID 15718104.
- ^ Maguire JJ, Parker WA, Foord SM, Bonner TI, Neubig RR, Davenport AP (March 2009). "International Union of Pharmacology. LXXII. Recommendations for trace amine receptor nomenclature". Pharmacological Reviews. 61 (1): 1–8. doi:10.1124/pr.109.001107. PMC 2830119. PMID 19325074.
- ^ Krause J (April 2008). "SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder". Expert Review of Neurotherapeutics. 8 (4): 611–25. doi:10.1586/14737175.8.4.611. PMID 18416663. S2CID 24589993.
Zinc binds at ... extracellular sites of the DAT [103], serving as a DAT inhibitor. In this context, controlled double-blind studies in children are of interest, which showed positive effects of zinc [supplementation] on symptoms of ADHD [105,106]. It should be stated that at this time [supplementation] with zinc is not integrated in any ADHD treatment algorithm.
- ^ Sulzer D (February 2011). "How addictive drugs disrupt presynaptic dopamine neurotransmission". Neuron. 69 (4): 628–49. doi:10.1016/j.neuron.2011.02.010. PMC 3065181. PMID 21338876.
They did not confirm the predicted straightforward relationship between uptake and release, but rather that some compounds including AMPH were better releasers than substrates for uptake. Zinc, moreover, stimulates efflux of intracellular [3H]DA despite its concomitant inhibition of uptake (Scholze et al., 2002).
- ^ a b Scholze P, Nørregaard L, Singer EA, Freissmuth M, Gether U, Sitte HH (June 2002). "The role of zinc ions in reverse transport mediated by monoamine transporters". The Journal of Biological Chemistry. 277 (24): 21505–13. doi:10.1074/jbc.M112265200. PMID 11940571.
The human dopamine transporter (hDAT) contains an endogenous high affinity Zn2+ binding site with three coordinating residues on its extracellular face (His193, His375, and Glu396). ... Although Zn2+ inhibited uptake, Zn2+ facilitated [3H]MPP+ release induced by amphetamine, MPP+, or K+-induced depolarization specifically at hDAT but not at the human serotonin and the norepinephrine transporter (hNET).
- ^ Scassellati C, Bonvicini C, Faraone SV, Gennarelli M (October 2012). "Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses". Journal of the American Academy of Child and Adolescent Psychiatry. 51 (10): 1003–1019.e20. doi:10.1016/j.jaac.2012.08.015. PMID 23021477.
With regard to zinc supplementation, a placebo controlled trial reported that doses up to 30 mg/day of zinc were safe for at least 8 weeks, but the clinical effect was equivocal except for the finding of a 37% reduction in amphetamine optimal dose with 30 mg per day of zinc.110
- ^ Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG (January 1999). "Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity". Science. 283 (5400): 397–401. Bibcode:1999Sci...283..397G. doi:10.1126/science.283.5400.397. PMID 9888856. S2CID 9629915.
- ^ Greenwood TA, Alexander M, Keck PE, McElroy S, Sadovnick AD, Remick RA, et al. (March 2001). "Evidence for linkage disequilibrium between the dopamine transporter and bipolar disorder". American Journal of Medical Genetics. 105 (2): 145–51. doi:10.1002/1096-8628(2001)9999:9999<::AID-AJMG1161>3.0.CO;2-8. PMID 11304827.
- ^ Yang B, Chan RC, Jing J, Li T, Sham P, Chen RY (June 2007). "A meta-analysis of association studies between the 10-repeat allele of a VNTR polymorphism in the 3'-UTR of dopamine transporter gene and attention deficit hyperactivity disorder". American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 144B (4): 541–50. doi:10.1002/ajmg.b.30453. PMID 17440978. S2CID 22881996.
- ^ Seymari A, Naseh A, Rezaei S, Salehi Z, Kousha M (January 2024). "The Relationship between Gene SLC6A3 Variable Number of Tandem Repeat (VNTR) and Attention-Deficit/Hyperactivity Disorder". Iranian Journal of Psychiatry. 19 (1): 99–106. doi:10.18502/ijps.v19i1.14345. PMC 10896761. PMID 38420272.
- ^ Sander T, Harms H, Podschus J, Finckh U, Nickel B, Rolfs A, et al. (February 1997). "Allelic association of a dopamine transporter gene polymorphism in alcohol dependence with withdrawal seizures or delirium". Biological Psychiatry. 41 (3): 299–304. doi:10.1016/S0006-3223(96)00044-3. PMID 9024952. S2CID 42947314.
- ^ Ueno S, Nakamura M, Mikami M, Kondoh K, Ishiguro H, Arinami T, et al. (November 1999). "Identification of a novel polymorphism of the human dopamine transporter (DAT1) gene and the significant association with alcoholism". Molecular Psychiatry. 4 (6): 552–7. doi:10.1038/sj.mp.4000562. PMID 10578237.
- ^ Ueno S (February 2003). "Genetic polymorphisms of serotonin and dopamine transporters in mental disorders". The Journal of Medical Investigation. 50 (1–2): 25–31. PMID 12630565.
- ^ Beaver KM, Wright JP, DeLisi M (September 2008). "Delinquent peer group formation: evidence of a gene x environment correlation". The Journal of Genetic Psychology. 169 (3): 227–44. doi:10.3200/GNTP.169.3.227-244. PMID 18788325. S2CID 46592146.
- ^ Florida State University (2 October 2008). "Specific Gene Found In Adolescent Men With Delinquent Peers". ScienceDaily. Retrieved 8 October 2008.
- ^ Laasonen-Balk T, Kuikka J, Viinamäki H, Husso-Saastamoinen M, Lehtonen J, Tiihonen J (June 1999). "Striatal dopamine transporter density in major depression". Psychopharmacology. 144 (3): 282–5. doi:10.1007/s002130051005. PMID 10435396. S2CID 32882588.
- ^ Ng J, Zhen J, Meyer E, Erreger K, Li Y, Kakar N, et al. (April 2014). "Dopamine transporter deficiency syndrome: phenotypic spectrum from infancy to adulthood". Brain. 137 (Pt 4): 1107–19. doi:10.1093/brain/awu022. PMC 3959557. PMID 24613933.
- ^ Rothman RB, Ananthan S, Partilla JS, Saini SK, Moukha-Chafiq O, Pathak V, et al. (June 2015). "Studies of the biogenic amine transporters 15. Identification of novel allosteric dopamine transporter ligands with nanomolar potency". The Journal of Pharmacology and Experimental Therapeutics. 353 (3): 529–38. doi:10.1124/jpet.114.222299. PMC 4429677. PMID 25788711.
- ^ Aggarwal S, Liu X, Rice C, Menell P, Clark PJ, Paparoidamis N, et al. (2019). "Identification of a Novel Allosteric Modulator of the Human Dopamine Transporter". ACS Chem Neurosci. 10 (8): 3718–3730. doi:10.1021/acschemneuro.9b00262. PMC 6703927. PMID 31184115.
- ^ Wersinger C, Sidhu A (April 2003). "Attenuation of dopamine transporter activity by alpha-synuclein". Neuroscience Letters. 340 (3): 189–92. doi:10.1016/S0304-3940(03)00097-1. PMID 12672538. S2CID 54381509.
- ^ Lee FJ, Liu F, Pristupa ZB, Niznik HB (April 2001). "Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis". FASEB Journal. 15 (6): 916–26. doi:10.1096/fj.00-0334com. PMID 11292651. S2CID 3406798.
- ^ Torres GE, Yao WD, Mohn AR, Quan H, Kim KM, Levey AI, et al. (April 2001). "Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1". Neuron. 30 (1): 121–34. doi:10.1016/S0896-6273(01)00267-7. PMID 11343649. S2CID 17318937.
- ^ Peck EG, Holleran KM, Curry AM, Holter KM, Estave PM, Sens JP, et al. (August 2024). "Synaptogyrin-3 Prevents Cocaine Addiction and Dopamine Deficits". bioRxiv. doi:10.1101/2024.07.27.605436. PMC 11361146. PMID 39211138.
- ^ Carneiro AM, Ingram SL, Beaulieu JM, Sweeney A, Amara SG, Thomas SM, et al. (August 2002). "The multiple LIM domain-containing adaptor protein Hic-5 synaptically colocalizes and interacts with the dopamine transporter". The Journal of Neuroscience. 22 (16): 7045–54. doi:10.1523/JNEUROSCI.22-16-07045.2002. PMC 6757888. PMID 12177201.
- ^ Midde NM, Yuan Y, Quizon PM, Sun WL, Huang X, Zhan CG, et al. (March 2015). "Mutations at tyrosine 88, lysine 92 and tyrosine 470 of human dopamine transporter result in an attenuation of HIV-1 Tat-induced inhibition of dopamine transport". Journal of Neuroimmune Pharmacology. 10 (1): 122–35. doi:10.1007/s11481-015-9583-3. PMC 4388869. PMID 25604666.
- ^ Midde NM, Huang X, Gomez AM, Booze RM, Zhan CG, Zhu J (September 2013). "Mutation of tyrosine 470 of human dopamine transporter is critical for HIV-1 Tat-induced inhibition of dopamine transport and transporter conformational transitions". Journal of Neuroimmune Pharmacology. 8 (4): 975–87. doi:10.1007/s11481-013-9464-6. PMC 3740080. PMID 23645138.
- ^ Purohit V, Rapaka R, Shurtleff D (August 2011). "Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia". Molecular Neurobiology. 44 (1): 102–10. doi:10.1007/s12035-011-8195-z. PMID 21717292. S2CID 13319355.
- ^ a b c d Hitri A, Hurd YL, Wyatt RJ, Deutsch SI (February 1994). "Molecular, functional and biochemical characteristics of the dopamine transporter: regional differences and clinical relevance". Clin Neuropharmacol. 17 (1): 1–22. doi:10.1097/00002826-199402000-00001. PMID 8149355.
- ^ a b c d e f g Schmitt KC, Rothman RB, Reith ME (July 2013). "Nonclassical pharmacology of the dopamine transporter: atypical inhibitors, allosteric modulators, and partial substrates". J Pharmacol Exp Ther. 346 (1): 2–10. doi:10.1124/jpet.111.191056. PMC 3684841. PMID 23568856.
- ^ a b c d e f g h i j k l Reith ME, Blough BE, Hong WC, Jones KT, Schmitt KC, Baumann MH, et al. (February 2015). "Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter". Drug Alcohol Depend. 147: 1–19. doi:10.1016/j.drugalcdep.2014.12.005. PMC 4297708. PMID 25548026.
- ^ Harsing LG, Knoll J, Miklya I (August 2022). "Enhancer Regulation of Dopaminergic Neurochemical Transmission in the Striatum". Int J Mol Sci. 23 (15): 8543. doi:10.3390/ijms23158543. PMC 9369307. PMID 35955676.
- ^ Kostrzewa RM (2022). "Survey of Selective Monoaminergic Neurotoxins Targeting Dopaminergic, Noradrenergic, and Serotoninergic Neurons". Handbook of Neurotoxicity. Cham: Springer International Publishing. pp. 159–198. doi:10.1007/978-3-031-15080-7_53. ISBN 978-3-031-15079-1.
- ^ Storch A, Ludolph AC, Schwarz J (October 2004). "Dopamine transporter: involvement in selective dopaminergic neurotoxicity and degeneration". J Neural Transm (Vienna). 111 (10–11): 1267–1286. doi:10.1007/s00702-004-0203-2. PMID 15480838.
- ^ Sotnikova TD, Beaulieu JM, Gainetdinov RR, Caron MG (February 2006). "Molecular biology, pharmacology and functional role of the plasma membrane dopamine transporter". CNS Neurol Disord Drug Targets. 5 (1): 45–56. doi:10.2174/187152706784111579. PMID 16613553.
- ^ Aceto MD, Botton I, Martin R, Levitt M, Bentley HC, Speight PT (1970). "Pharmacologic properties and mechanism of action of amfonelic acid". Eur J Pharmacol. 10 (3): 344–354. doi:10.1016/0014-2999(70)90206-2. PMID 4393073.
- ^ Porrino LJ, Goodman NL, Sharpe LG (November 1988). "Intravenous self-administration of the indirect dopaminergic agonist amfonelic acid by rats". Pharmacol Biochem Behav. 31 (3): 623–626. doi:10.1016/0091-3057(88)90240-7. PMID 2908003.
- ^ Garattini S (July 1997). "Pharmacology of amineptine, an antidepressant agent acting on the dopaminergic system: a review". Int Clin Psychopharmacol. 12 (Suppl 3): S15–S19. doi:10.1097/00004850-199707003-00003. PMID 9347388.
- ^ Bonnet JJ, Chagraoui A, Protais P, Costentin J (1987). "Interactions of amineptine with the neuronal dopamine uptake system: neurochemical in vitro and in vivo studies". J Neural Transm. 69 (3–4): 211–220. doi:10.1007/BF01244342. PMID 3625193.
- ^ Evans EA, Sullivan MA (2014). "Abuse and misuse of antidepressants". Subst Abuse Rehabil. 5: 107–120. doi:10.2147/SAR.S37917. PMC 4140701. PMID 25187753.
- ^ Haddad P (1999). "Do antidepressants have any potential to cause addiction?". J Psychopharmacol. 13 (3): 300–307. doi:10.1177/026988119901300321. PMID 10512092.
- ^ Dutta AK, Zhang S, Kolhatkar R, Reith ME (October 2003). "Dopamine transporter as target for drug development of cocaine dependence medications". Eur J Pharmacol. 479 (1–3): 93–106. doi:10.1016/j.ejphar.2003.08.060. PMID 14612141.
- ^ Pergolizzi J, Breve F, Magnusson P, LeQuang JA, Varrassi G (February 2022). "Cocaethylene: When Cocaine and Alcohol Are Taken Together". Cureus. 14 (2): e22498. doi:10.7759/cureus.22498. PMC 8956485. PMID 35345678.
- ^ Landry MJ (1992). "An overview of cocaethylene, an alcohol-derived, psychoactive, cocaine metabolite". J Psychoactive Drugs. 24 (3): 273–276. doi:10.1080/02791072.1992.10471648. PMID 1432406.
- ^ a b c d e f g h i j k l m n o p q Heal DJ, Gosden J, Smith SL (December 2014). "Dopamine reuptake transporter (DAT) "inverse agonism"--a novel hypothesis to explain the enigmatic pharmacology of cocaine". Neuropharmacology. 87: 19–40. doi:10.1016/j.neuropharm.2014.06.012. PMID 24953830.
- ^ a b c d e f g Aggarwal S, Mortensen OV (2023). "Discovery and Development of Monoamine Transporter Ligands". Drug Development in Psychiatry. Advances in Neurobiology. Vol. 30. pp. 101–129. doi:10.1007/978-3-031-21054-9_4. ISBN 978-3-031-21053-2. PMC 10074400. PMID 36928847.
- ^ a b c Tanda G, Hersey M, Hempel B, Xi ZX, Newman AH (February 2021). "Modafinil and its structural analogs as atypical dopamine uptake inhibitors and potential medications for psychostimulant use disorder". Curr Opin Pharmacol. 56: 13–21. doi:10.1016/j.coph.2020.07.007. PMC 8247144. PMID 32927246.
- ^ Newman AH, Ku T, Jordan CJ, Bonifazi A, Xi ZX (January 2021). "New Drugs, Old Targets: Tweaking the Dopamine System to Treat Psychostimulant Use Disorders". Annu Rev Pharmacol Toxicol. 61: 609–628. doi:10.1146/annurev-pharmtox-030220-124205. PMC 9341034. PMID 33411583.
- ^ Glennon RA, Young R (September 2016). "Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP)". Brain Res Bull. 126 (Pt 1): 111–126. doi:10.1016/j.brainresbull.2016.04.011. PMC 5817884. PMID 27142261.
- ^ Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. (March 2013). "Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive 'bath salts' products". Neuropsychopharmacology. 38 (4): 552–562. doi:10.1038/npp.2012.204. PMC 3572453. PMID 23072836.
- ^ a b c d e f g h Nepal B, Das S, Reith ME, Kortagere S (2023). "Overview of the structure and function of the dopamine transporter and its protein interactions". Front Physiol. 14: 1150355. doi:10.3389/fphys.2023.1150355. PMC 10020207. PMID 36935752.
- ^ Cheng MH, Block E, Hu F, Cobanoglu MC, Sorkin A, Bahar I (2015). "Insights into the Modulation of Dopamine Transporter Function by Amphetamine, Orphenadrine, and Cocaine Binding". Front Neurol. 6: 134. doi:10.3389/fneur.2015.00134. PMC 4460958. PMID 26106364.
- ^ Katz JL, Newman AH, Izenwasser S (July 1997). "Relations between heterogeneity of dopamine transporter binding and function and the behavioral pharmacology of cocaine". Pharmacol Biochem Behav. 57 (3): 505–512. doi:10.1016/s0091-3057(96)00441-8. PMID 9218275.
- ^ Bergman J, France CP, Holtzman SG, Katz JL, Koek W, Stephens DN (December 2000). "Agonist efficacy, drug dependence, and medications development: preclinical evaluation of opioid, dopaminergic, and GABAA-ergic ligands". Psychopharmacology (Berl). 153 (1): 67–84. doi:10.1007/s002130000567. PMID 11255930.
- ^ Izenwasser S, Newman AH, Cox BM, Katz JL (February 1996). "The cocaine-like behavioral effects of meperidine are mediated by activity at the dopamine transporter". Eur J Pharmacol. 297 (1–2): 9–17. doi:10.1016/0014-2999(95)00696-6. PMID 8851160.
- ^ White MW, Archer JR (2013). "Pipradrol and Pipradrol Derivatives". Novel Psychoactive Substances. Elsevier. pp. 233–259. doi:10.1016/b978-0-12-415816-0.00010-9. ISBN 978-0-12-415816-0.
- ^ a b c Golovko AI, Bonitenko EY, Ivanov MB, Barinov VA, Zatsepin EP (2016). "The neurochemical bases of the pharmacological activity of ligands of monoamine-transport systems". Neurochemical Journal. 10 (3): 173–183. doi:10.1134/S1819712416030065. ISSN 1819-7124.
- ^ a b Hersey M, Bacon AK, Bailey LG, Coggiano MA, Newman AH, Leggio L, et al. (2021). "Psychostimulant Use Disorder, an Unmet Therapeutic Goal: Can Modafinil Narrow the Gap?". Front Neurosci. 15: 656475. doi:10.3389/fnins.2021.656475. PMC 8187604. PMID 34121988.
- ^ Hauck Newman A, Katz JL (2008). "Atypical Dopamine Uptake Inhibitors that Provide Clues About Cocaine's Mechanism at the Dopamine Transporter". Transporters as Targets for Drugs. Topics in Medicinal Chemistry. Vol. 4. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 95–129. doi:10.1007/7355_2008_027. ISBN 978-3-540-87911-4.
- ^ a b c Rothman RB (1994). "A Review of the Effects of Dopaminergic Agents in Humans: Implications for Medication Development". In Erinoff L, Brown RM (eds.). Neurobiological Models for Evaluating Mechanisms Underlying Cocaine Addiction (NIDA Research Monograph 145). U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Drug Abuse. pp. 67–87. Retrieved 4 August 2024.
- ^ Naglich AC, Brown ES, Adinoff B (2019). "Systematic review of preclinical, clinical, and post-marketing evidence of bupropion misuse potential". Am J Drug Alcohol Abuse. 45 (4): 341–354. doi:10.1080/00952990.2018.1545023. PMID 30601027.
- ^ Noe G, Shah K, Ongchuan S, Munjal S (2024). "Clinical Presentations of Bupropion Prescription Drug Misuse: A Systematic Review". J Clin Psychopharmacol. 44 (3): 284–290. doi:10.1097/JCP.0000000000001858. PMID 38656298.
- ^ Aikoye S, Basiru TO, Nwoye I, Adereti I, Asuquo S, Ezeokoli A, et al. (March 2023). "A Systematic Review of Abuse or Overprescription of Bupropion in American Prisons and a Synthesis of Case Reports on Bupropion Abuse in American Prison and Non-prison Systems". Cureus. 15 (3): e36189. doi:10.7759/cureus.36189. PMC 10104426. PMID 37065297.
- ^ Costa R, Oliveira NG, Dinis-Oliveira RJ (August 2019). "Pharmacokinetic and pharmacodynamic of bupropion: integrative overview of relevant clinical and forensic aspects". Drug Metab Rev. 51 (3): 293–313. doi:10.1080/03602532.2019.1620763. PMID 31124380.
- ^ Jordan CJ, Cao J, Newman AH, Xi ZX (November 2019). "Progress in agonist therapy for substance use disorders: Lessons learned from methadone and buprenorphine". Neuropharmacology. 158: 107609. doi:10.1016/j.neuropharm.2019.04.015. PMC 6745247. PMID 31009632.
- ^ a b Hersey M, Bartole MK, Jones CS, Newman AH, Tanda G (July 2023). "Are There Prevalent Sex Differences in Psychostimulant Use Disorder? A Focus on the Potential Therapeutic Efficacy of Atypical Dopamine Uptake Inhibitors". Molecules. 28 (13): 5270. doi:10.3390/molecules28135270. PMC 10343811. PMID 37446929.
- ^ a b c Rothman RB, Glowa JR (1995). "A review of the effects of dopaminergic agents on humans, animals, and drug-seeking behavior, and its implications for medication development. Focus on GBR 12909". Mol Neurobiol. 11 (1–3): 1–19. doi:10.1007/BF02740680. PMID 8561954.
- ^ a b c Salamone JD, Correa M (January 2024). "The Neurobiology of Activational Aspects of Motivation: Exertion of Effort, Effort-Based Decision Making, and the Role of Dopamine". Annu Rev Psychol. 75: 1–32. doi:10.1146/annurev-psych-020223-012208. hdl:10234/207207. PMID 37788571.
- ^ Hersey M, Tanda G (2024). "Modafinil, an atypical CNS stimulant?". Pharmacological Advances in Central Nervous System Stimulants. Adv Pharmacol. Vol. 99. pp. 287–326. doi:10.1016/bs.apha.2023.10.006. ISBN 978-0-443-21933-7. PMID 38467484.
- ^ Böning J, Fuchs G (September 1986). "Nomifensine and psychological dependence--a case report". Pharmacopsychiatry. 19 (5): 386–388. doi:10.1055/s-2007-1017275. PMID 3774872.
- ^ Veinberg G, Vavers E, Orlova N, Kuznecovs J, Domracheva I, Vorona M, et al. (2015). "Stereochemistry of phenylpiracetam and its methyl derivative: improvement of the pharmacological profile". Chemistry of Heterocyclic Compounds. 51 (7): 601–606. doi:10.1007/s10593-015-1747-9. ISSN 0009-3122.
Phenylpiracetam was originally designed as a nootropic drug for the sustenance and improvement of the physical condition and cognition abilities of Soviet space crews.2 Later, especially during the last decade, phenylpiracetam was introduced into general clinical practice in Russia and in some Eastern European countries. The possible target receptors and mechanisms for the acute activity of this drug remained unclear, until very recently it was found that (R)-phenylpiracetam (5) (MRZ-9547) is a selective dopamine transporter inhibitor that moderately stimulates striatal dopamine release.19
- ^ a b Sommer S, Danysz W, Russ H, Valastro B, Flik G, Hauber W (December 2014). "The dopamine reuptake inhibitor MRZ-9547 increases progressive ratio responding in rats". The International Journal of Neuropsychopharmacology. 17 (12): 2045–2056. doi:10.1017/S1461145714000996. PMID 24964269.
Here, we tested the effects of MRZ-9547 [...], and its l-enantiomer MRZ-9546 on effort-related decision making in rats. The racemic form of these compounds referred to as phenotropil has been shown to stimulate motor activity in rats (Zvejniece et al., 2011) and enhance physical capacity and cognition in humans (Malykh and Sadaie, 2010). [...] MRZ-9547 turned out to be a DAT inhibitor as shown by displacement of binding of [125I] RTI-55 (IC50 = 4.82 ± 0.05 μM, n=3) to human recombinant DAT expressed in CHO-K1 cells and inhibition of DA uptake (IC50 = 14.5 ± 1.6 μM, n=2) in functional assays in the same cells. It inhibited norepinephrine transporter (NET) with an IC50 of 182 μM (one experiment in duplicate). The potencies for the l-enantiomer MRZ-9546 were as follows: DAT binding (Ki = 34.8 ± 14.8 μM, n=3), DAT function (IC50 = 65.5 ± 8.3 μM, n=2) and NET function (IC50 = 667 μM, one experiment performed in duplicate).
- ^ Zvejniece L, Svalbe B, Vavers E, Makrecka-Kuka M, Makarova E, Liepins V, et al. (September 2017). "S-phenylpiracetam, a selective DAT inhibitor, reduces body weight gain without influencing locomotor activity". Pharmacology, Biochemistry, and Behavior. 160: 21–29. doi:10.1016/j.pbb.2017.07.009. PMID 28743458. S2CID 13658335.
- ^ Stutz PV, Golani LK, Witkin JM (February 2019). "Animal models of fatigue in major depressive disorder". Physiology & Behavior. 199: 300–305. doi:10.1016/j.physbeh.2018.11.042. PMID 30513290.
In a study performed by Sommer et al. (2014), healthy rats treated with the selective dopamine transport (DAT) inhibitor MRZ-9547 (Fig. 1) chose high effort, high reward more often than their untreated matched controls. Unlike similar studies, however, depressive symptoms were not induced before treatment; rather, baseline healthy controls were compared to healthy rats treated with MRZ-9547. [...] In one study, the selective DAT inhibitor MRZ-9547 increased the number of lever presses more than untreated controls (Sommer et al., 2014). The investigators concluded that such effort-based "decision making in rodents could provide an animal model for motivational dysfunctions related to effort expenditure such as fatigue, e.g. in Parkinson's disease or major depression." Based upon the findings with MRZ-9547, they suggested that this drug mechanism might be a valuable therapeutic entity for fatigue in neurological and neuropsychiatric disorders. [...] A high effort bias been reported with bupropion (Randall et al., 2015), lisdexamfetamine (Yohn etal., 2016e), and the DA uptake blockers MRZ-9547 (Sommer et al., 2014), PRX-14040 (Fig. 1) (Yohn et al., 2016d) and GBR12909 (Fig. 1) (Yohn et al., 2016c).
- ^ Dekundy A, Mela F, Hofmann M, Danysz W (June 2015). "Effects of dopamine uptake inhibitor MRZ-9547 in animal models of Parkinson's disease". Journal of Neural Transmission. 122 (6): 809–818. doi:10.1007/s00702-014-1326-8. PMID 25319446.
- ^ Ngo Q, Plante DT (19 September 2022). "An Update on the Misuse and Abuse Potential of Pharmacological Treatments for Central Disorders of Hypersomnolence". Current Sleep Medicine Reports. 8 (4): 147–159. doi:10.1007/s40675-022-00227-4. ISSN 2198-6401.
- ^ Yang J, Gao J (August 2019). "Solriamfetol for the treatment of excessive daytime sleepiness associated with narcolepsy". Expert Rev Clin Pharmacol. 12 (8): 723–728. doi:10.1080/17512433.2019.1632705. PMID 31215815.
- ^ Mikelman SR, Guptaroy B, Schmitt KC, Jones KT, Zhen J, Reith ME, et al. (October 2018). "Tamoxifen Directly Interacts with the Dopamine Transporter". J Pharmacol Exp Ther. 367 (1): 119–128. doi:10.1124/jpet.118.248179. PMC 7250473. PMID 30108161.
- ^ Schoedel KA, Meier D, Chakraborty B, Manniche PM, Sellers EM (July 2010). "Subjective and objective effects of the novel triple reuptake inhibitor tesofensine in recreational stimulant users". Clin Pharmacol Ther. 88 (1): 69–78. doi:10.1038/clpt.2010.67. PMID 20520602.
- ^ a b c d e f g h Rothman RB, Baumann MH (October 2003). "Monoamine transporters and psychostimulant drugs". Eur J Pharmacol. 479 (1–3): 23–40. doi:10.1016/j.ejphar.2003.08.054. PMID 14612135.
- ^ a b c d e f g Rothman RB, Baumann MH (2006). "Therapeutic potential of monoamine transporter substrates". Curr Top Med Chem. 6 (17): 1845–1859. doi:10.2174/156802606778249766. PMID 17017961.
- ^ Salamone JD, Correa M, Ferrigno S, Yang JH, Rotolo RA, Presby RE (October 2018). "The Psychopharmacology of Effort-Related Decision Making: Dopamine, Adenosine, and Insights into the Neurochemistry of Motivation". Pharmacol Rev. 70 (4): 747–762. doi:10.1124/pr.117.015107. PMC 6169368. PMID 30209181.
- ^ Desibhatla M (16 June 2021). "The Development and Evaluation of Novel DA Transport Inhibitors and their Effects on Effort-Related Motivation: A Review". Honors Scholar Theses. Retrieved 10 August 2024.
- ^ Yohn SE, Gogoj A, Haque A, Lopez-Cruz L, Haley A, Huxley P, et al. (September 2016). "Evaluation of the effort-related motivational effects of the novel dopamine uptake inhibitor PRX-14040". Pharmacol Biochem Behav. 148: 84–91. doi:10.1016/j.pbb.2016.06.004. PMID 27296079.
- ^ Pinterova N, Horsley RR, Palenicek T (2017). "Synthetic Aminoindanes: A Summary of Existing Knowledge". Front Psychiatry. 8: 236. doi:10.3389/fpsyt.2017.00236. PMC 5698283. PMID 29204127.
- ^ Simmler LD, Rickli A, Schramm Y, Hoener MC, Liechti ME (March 2014). "Pharmacological profiles of aminoindanes, piperazines, and pipradrol derivatives" (PDF). Biochem Pharmacol. 88 (2): 237–244. doi:10.1016/j.bcp.2014.01.024. PMID 24486525.
- ^ a b Blough BE, Landavazo A, Partilla JS, Decker AM, Page KM, Baumann MH, et al. (October 2014). "Alpha-ethyltryptamines as dual dopamine-serotonin releasers". Bioorganic & Medicinal Chemistry Letters. 24 (19): 4754–4758. doi:10.1016/j.bmcl.2014.07.062. PMC 4211607. PMID 25193229.
- ^ Banks ML, Bauer CT, Blough BE, et al. (June 2014). "Abuse-related effects of dual dopamine/serotonin releasers with varying potency to release norepinephrine in male rats and rhesus monkeys". Exp Clin Psychopharmacol. 22 (3): 274–284. doi:10.1037/a0036595. PMC 4067459. PMID 24796848.
- ^ Glennon RA, Dukat MG (December 2023). "α-Ethyltryptamine: A Ratiocinatory Review of a Forgotten Antidepressant". ACS Pharmacology & Translational Science. 6 (12): 1780–1789. doi:10.1021/acsptsci.3c00139. PMC 10714429. PMID 38093842.
- ^ Oeri HE (May 2021). "Beyond ecstasy: Alternative entactogens to 3,4-methylenedioxymethamphetamine with potential applications in psychotherapy". Journal of Psychopharmacology. 35 (5): 512–536. doi:10.1177/0269881120920420. PMC 8155739. PMID 32909493.
- ^ a b Blough BE, Landavazo A, Partilla JS, Decker AM, Page KM, Baumann MH, et al. (October 2014). "Alpha-ethyltryptamines as dual dopamine-serotonin releasers". Bioorganic & Medicinal Chemistry Letters. 24 (19): 4754–4758. doi:10.1016/j.bmcl.2014.07.062. PMC 4211607. PMID 25193229.
- ^ a b c Rothman RB, Baumann MH (December 2005). "Targeted screening for biogenic amine transporters: potential applications for natural products". Life Sci. 78 (5): 512–518. doi:10.1016/j.lfs.2005.09.001. PMID 16202429.
- ^ a b c Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, et al. (October 2003). "In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates". J Pharmacol Exp Ther. 307 (1): 138–145. doi:10.1124/jpet.103.053975. PMID 12954796.
- ^ Hutson PH, Pennick M, Secker R (December 2014). "Preclinical pharmacokinetics, pharmacology and toxicology of lisdexamfetamine: a novel d-amphetamine pro-drug". Neuropharmacology. 87: 41–50. doi:10.1016/j.neuropharm.2014.02.014. PMID 24594478.
- ^ Heal DJ, Cheetham SC, Smith SL (December 2009). "The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety". Neuropharmacology. 57 (7–8): 608–618. doi:10.1016/j.neuropharm.2009.08.020. PMID 19761781.
- ^ Green AR, King MV, Shortall SE, Fone KC (May 2014). "The preclinical pharmacology of mephedrone; not just MDMA by another name". Br J Pharmacol. 171 (9): 2251–2268. doi:10.1111/bph.12628. PMC 3997268. PMID 24654568.
- ^ Simmons SJ, Leyrer-Jackson JM, Oliver CF, Hicks C, Muschamp JW, Rawls SM, et al. (October 2018). "DARK Classics in Chemical Neuroscience: Cathinone-Derived Psychostimulants". ACS Chem Neurosci. 9 (10): 2379–2394. doi:10.1021/acschemneuro.8b00147. PMC 6197900. PMID 29714473.
- ^ a b Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. (April 2012). "The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue". Neuropsychopharmacology. 37 (5): 1192–1203. doi:10.1038/npp.2011.304. PMC 3306880. PMID 22169943.
- ^ Rothman RB, Blough BE, Baumann MH (January 2007). "Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions". AAPS J. 9 (1): E1–10. doi:10.1208/aapsj0901001. PMC 2751297. PMID 17408232.
- ^ a b c Parker EM, Cubeddu LX (April 1988). "Comparative effects of amphetamine, phenylethylamine and related drugs on dopamine efflux, dopamine uptake and mazindol binding". J Pharmacol Exp Ther. 245 (1): 199–210. PMID 3129549.
- ^ Patrick KS, Markowitz JS (November 1997). "Pharmacology of methylphenidate, amphetamine enantiomers and pemoline in attention-deficit hyperactivity disorder". Human Psychopharmacology: Clinical and Experimental. 12 (6): 527–546. doi:10.1002/(SICI)1099-1077(199711/12)12:6<527::AID-HUP932>3.0.CO;2-U. eISSN 1099-1077. ISSN 0885-6222. S2CID 144548631.
- ^ Nishino S, Mignot E (May 1997). "Pharmacological aspects of human and canine narcolepsy". Prog Neurobiol. 52 (1): 27–78. doi:10.1016/s0301-0082(96)00070-6. PMID 9185233. S2CID 31839355.
- ^ Fuller RW, Perry KW, Bymaster FP, Wong DT (March 1978). "Comparative effects of pemoline, amfonelic acid and amphetamine on dopamine uptake and release in vitro and on brain 3,4-dihydroxyphenylacetic acid concentration in spiperone-treated rats". The Journal of Pharmacy and Pharmacology. 30 (3): 197–198. doi:10.1111/j.2042-7158.1978.tb13201.x. PMID 24701.
- ^ Blough BE, Landavazo A, Decker AM, Partilla JS, Baumann MH, Rothman RB (October 2014). "Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes". Psychopharmacology. 231 (21): 4135–4144. doi:10.1007/s00213-014-3557-7. PMC 4194234. PMID 24800892.
- ^ a b Zhao G, Qin GW, Wang J, Chu WJ, Guo LH (January 2010). "Functional activation of monoamine transporters by luteolin and apigenin isolated from the fruit of Perilla frutescens (L.) Britt". Neurochem Int. 56 (1): 168–176. doi:10.1016/j.neuint.2009.09.015. PMID 19815045.
- ^ Zhang J, Liu X, Lei X, Wang L, Guo L, Zhao G, et al. (November 2010). "Discovery and synthesis of novel luteolin derivatives as DAT agonists". Bioorg Med Chem. 18 (22): 7842–7848. doi:10.1016/j.bmc.2010.09.049. PMID 20971650.
- ^ Rothman RB, Dersch CM, Carroll FI, Ananthan S (March 2002). "Studies of the biogenic amine transporters. VIII: identification of a novel partial inhibitor of dopamine uptake and dopamine transporter binding". Synapse. 43 (4): 268–274. doi:10.1002/syn.10046. PMID 11835522.
- ^ a b Pariser JJ, Partilla JS, Dersch CM, Ananthan S, Rothman RB (July 2008). "Studies of the biogenic amine transporters. 12. Identification of novel partial inhibitors of amphetamine-induced dopamine release". J Pharmacol Exp Ther. 326 (1): 286–295. doi:10.1124/jpet.108.139675. PMC 2562894. PMID 18441249.
- ^ a b Rothman RB, Dersch CM, Ananthan S, Partilla JS (May 2009). "Studies of the biogenic amine transporters. 13. Identification of "agonist" and "antagonist" allosteric modulators of amphetamine-induced dopamine release". J Pharmacol Exp Ther. 329 (2): 718–728. doi:10.1124/jpet.108.149088. PMC 2672863. PMID 19244097.
- ^ Macolino-Kane CM, Ciallella JR, Lipinski CA, Reaume AG (14 July 2017). "Phenotypic Screening". Drug Repositioning. Frontiers in Neurotherapeutics. Boca Raton: CRC Press. pp. 121–145. doi:10.4324/9781315373669-7. ISBN 978-1-315-37366-9.
- ^ Al'tshuler RA (2005). "Comparative Molecular Model Estimation of the Affinity of Phenylethylamines to the Binding Sites of Membrane Transporters". Pharmaceutical Chemistry Journal. 39 (4): 169–175. doi:10.1007/s11094-005-0110-3. ISSN 0091-150X.
- ^ Bulling S, Schicker K, Zhang YW, Steinkellner T, Stockner T, Gruber CW, et al. (May 2012). "The mechanistic basis for noncompetitive ibogaine inhibition of serotonin and dopamine transporters". J Biol Chem. 287 (22): 18524–18534. doi:10.1074/jbc.M112.343681. PMC 3365767. PMID 22451652.
- ^ Harsing LG, Sershen H, Lajtha A (1994). "Evidence that ibogaine releases dopamine from the cytoplasmic pool in isolated mouse striatum". J Neural Transm Gen Sect. 96 (3): 215–225. doi:10.1007/BF01294788. PMID 7826572.
- ^ Wells GB, Lopez MC, Tanaka JC (April 1999). "The effects of ibogaine on dopamine and serotonin transport in rat brain synaptosomes". Brain Res Bull. 48 (6): 641–647. doi:10.1016/s0361-9230(99)00053-2. PMID 10386845.
- ^ a b Nguyen H, Cheng MH, Lee JY, Aggarwal S, Mortensen OV, Bahar I (2024). "Allosteric modulation of serotonin and dopamine transporters: New insights from computations and experiments". Curr Res Physiol. 7: 100125. doi:10.1016/j.crphys.2024.100125. PMC 11148570. PMID 38836245.
- ^ Aggarwal S, Liu X, Rice C, Menell P, Clark PJ, Paparoidamis N, et al. (August 2019). "Identification of a Novel Allosteric Modulator of the Human Dopamine Transporter". ACS Chem Neurosci. 10 (8): 3718–3730. doi:10.1021/acschemneuro.9b00262. PMC 6703927. PMID 31184115.
- ^ Aggarwal S, Cheng MH, Salvino JM, Bahar I, Mortensen OV (June 2021). "Functional Characterization of the Dopaminergic Psychostimulant Sydnocarb as an Allosteric Modulator of the Human Dopamine Transporter". Biomedicines. 9 (6): 634. doi:10.3390/biomedicines9060634. PMC 8227285. PMID 34199621.
- ^ Tosh DK, Janowsky A, Eshleman AJ, Warnick E, Gao ZG, Chen Z, et al. (April 2017). "Scaffold Repurposing of Nucleosides (Adenosine Receptor Agonists): Enhanced Activity at the Human Dopamine and Norepinephrine Sodium Symporters". J Med Chem. 60 (7): 3109–3123. doi:10.1021/acs.jmedchem.7b00141. PMC 5501184. PMID 28319392.
- ^ Rothman RB, Ananthan S, Partilla JS, Saini SK, Moukha-Chafiq O, Pathak V, et al. (June 2015). "Studies of the biogenic amine transporters 15. Identification of novel allosteric dopamine transporter ligands with nanomolar potency". J Pharmacol Exp Ther. 353 (3): 529–538. doi:10.1124/jpet.114.222299. PMC 4429677. PMID 25788711.
- ^ Moerke MJ, Ananthan S, Banks ML, Eltit JM, Freitas KC, Johnson AR, et al. (November 2018). "Interactions between Cocaine and the Putative Allosteric Dopamine Transporter Ligand SRI-31142". J Pharmacol Exp Ther. 367 (2): 222–233. doi:10.1124/jpet.118.250902. PMC 6170971. PMID 30150482.
External links
[edit]- Dopamine transporter-related Associations, Experiments, Publications and Clinical Trials
- Dopamine+Transporter at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: Q7K4Y6 (Drosophila melanogaster Sodium-dependent dopamine transporter) at the PDBe-KB.